关于先锋
Company Profile
Warmly congratulate Anhui Pioneer Pharmaceutical Fenofibrate Capsules for being the first to pass the consistency evaluation of generic drugs
Release time:
2023-02-25
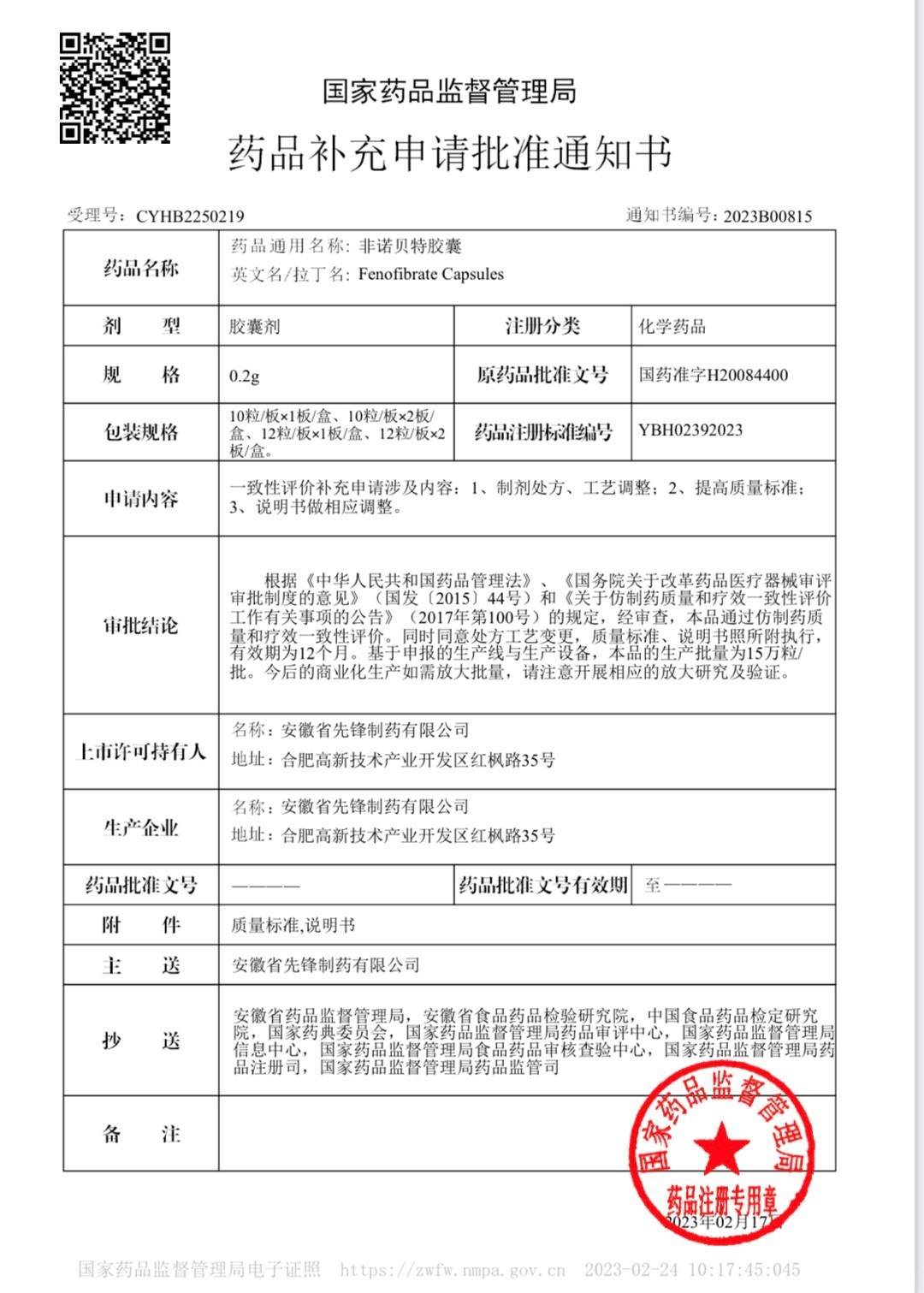
On February 24, 2023, Anhui Pioneer Pharmaceutical Co., Ltd. (hereinafter referred to as "Pioneer Pharmaceutical") ushered in the approval and issuance of the approval document for the consistency evaluation of generic drugs this year. The State Drug Administration approved and issued the "Drug Supplementary Application Approval Document", officially notifying Anhui Pioneer Pharmaceuticals that "Fenofibrate Capsules" is the first to pass the consistency evaluation of generic drug quality and efficacy!
On February 24, 2023, Anhui Pioneer Pharmaceutical Co., Ltd. (hereinafter referred to as "Pioneer Pharmaceutical") ushered in the approval and issuance of the approval document for the consistency evaluation of generic drugs this year. The State Drug Administration approved and issued the "Drug Supplementary Application Approval Document", officially notifying Anhui Pioneer Pharmaceuticals that "Fenofibrate Capsules" is the first to pass the consistency evaluation of generic drug quality and efficacy!

Fenofibrate capsules are a safe and highly effective fibrate lipid-lowering drug. National survey results show that the prevalence of adult hypercholesterolemia is 4.9%; the prevalence of hypertriglyceridemia is as high as 13.1%; the prevalence of low HDL-C blood is as high as 33.9%. The overall prevalence of dyslipidemia in Chinese adults is as high as 40.40%, and the number of people with dyslipidemia in China is as high as 500 million. Therefore, fenofibrate capsules are more suitable lipid-lowering drugs for Chinese people with hyperlipidemia!
Fenofibrate capsule is a typical peroxisome proliferator-activated receptor α (PPARα) agonist, which can increase triglyceride catabolism and can significantly reduce cholesterol (TC decreases by 20-25%), triglyceride ( TG decreased by 40% to 50%) and low-density lipoprotein (LDL-c decreased by 29%), high-density lipoprotein (HDL-ch increased by 40%), and uric acid was decreased (25%). For obese diabetic patients, fenofibrate can also reduce blood sugar while lowering blood lipids. Some studies have pointed out that oral administration of fenofibrate for 3 weeks can reduce fasting blood sugar, 2h postprandial blood sugar, and increase insulin sensitivity index. high. Clinically, it is mainly used to treat dyslipidemia characterized by elevated triglycerides and decreased high-density lipoprotein, and it can also fight against the occurrence and development of atherosclerosis through non-lipid-regulating effects. The product can be used as the first choice for patients with hyperlipidemia, type 2 diabetes and metabolic syndrome!
Features:
National medical insurance category B, 685 essential drug catalog varieties
Unique micronization process High bioavailability and good absorption
Enhance insulin sensitivity, more suitable for patients with type 2 diabetes and metabolic syndrome
Lower triglycerides, cholesterol, low-density lipoprotein, very low-density lipoprotein, increase high-density lipoprotein, comprehensively regulate lipids, reduce the occurrence of vascular lesions, and is the first choice for clinical treatment of hypertriglyceridemia!
According to relevant national policies, the quality and curative effect of drugs that pass the consistency evaluation are equivalent to the original research products, and will be properly supported in terms of medical insurance payment. Medical institutions should give priority to purchase and use them in clinical practice. The evaluation is conducive to improving the market competitiveness of the drug, and at the same time, it has accumulated valuable experience for the follow-up consistency evaluation product research and even the development of generic drugs of Pioneer Pharmaceuticals, and further accelerated the research and development, review, and approval of other consistency evaluation products and generic drugs. Anhui Pioneer Pharmaceutical Fenofibrate Capsules, as the first product to be reviewed, will definitely bring higher promotion value to the majority of partners. Welcome new and old customers to strengthen cooperation!
Related news
Contact us
Add:No.35 HongFeng Road, Hefei, Anhui, China.
Tel:86-551-65355028
Fax:86-551-65355022
Email:xfcn@xfcn.com

Follow us
Copyright © 2023 Anhui Pioneer Pharmaceutical Co., Ltd 皖ICP备2020016893号 Powered by:www.300.cn SEO




 86-551-65355028
86-551-65355028