关于先锋
Company Profile
08
2023-03
On March 7, 2023, when Goddess Day is approaching, Pioneer Pharmaceuticals welcomes entrepreneur friends from the fourth class committee of the 39th Cheung Kong Graduate School of Business to come to Pioneer to guide the work!
2023-03-08
27
2023-02
On the occasion of the New Year's Day in 2023 , Anhui Pioneer Pharmaceutical Co., Ltd. received two notices of approval for registration of generic drugs issued by the State Food and Drug Administration. The approved product is Clindamycin Phosphate Injection (Trademark: Xinruijia® ) with specifications of 2ml :0.3g and 4ml:0.6g ), and the approval numbers are Guoyao Zhunzi H20223952 and Guoyao Zhunzi H20223953 respectively . At the beginning of the new year, Pioneer Pharmaceuticals successfully obtained 2 R&D achievements, which will definitely have far-reaching significance for the long-term development of the company!
2023-02-27
25
2023-02
On February 24, 2023, Anhui Pioneer Pharmaceutical Co., Ltd. (hereinafter referred to as "Pioneer Pharmaceutical") ushered in the approval and issuance of the approval document for the consistency evaluation of generic drugs this year. The State Drug Administration approved and issued the "Drug Supplementary Application Approval Document", officially notifying Anhui Pioneer Pharmaceuticals that "Fenofibrate Capsules" is the first to pass the consistency evaluation of generic drug quality and efficacy!
2023-02-25
24
2020-09
Cooperation in the field of big health
On Wednesday, September 23, 2020, cooperative customers in the field of general health visited Pioneer, accompanied by Chairman Chen Dong, Vice President of Sales He Chuanlan, Sales Director Gao Lixia, Marketing Director Li Xiaobao, etc. Customers have fully recognized Pioneer's product technology and quality standard system, as well as the after-sales system. In the later stage, we will explore new models together and strengthen cooperation.
2020-09-24
08
2020-09
The board of directors of Pioneer Pharmaceuticals visited a number of pharmaceutical companies and reached strategic cooperation At the end of August 2020, Chairman Chen and General Manager Zheng Jian of Pioneer Pharmaceuticals visited pharmaceutical companies in Zhongshan, Zhuhai, Shenzhen, Chengdu and other places, and had in-depth communication with the heads of various companies on matters such as company listing and generic drug research and development. , and reached a number of strategic cooperation.
2020-09-08
02
2020-09
Good news! Schubert ® Fenofibrate capsule is launched
good news! Shubeite® Fenofibrate Capsules Launched Fenofibrate Capsules belong to the third generation of fibrate lipid-lowering drugs, and are mainly used clinically to treat elevated triglyceride (TG), elevated very low-density lipoprotein (VLD), low-density lipoprotein cholesterol (LDL- C) Dyslipidemia characterized by elevated and decreased high-density lipoprotein (HDL). By activating peroxisome proliferator-activated receptor α to play a protective role in blood vessels, it can significantly reduce the risk of microvascular complications in patients with hyperlipidemia, metabolic syndrome, and type 2 diabetes. Micronized technology, uniform release, regular absorption, greatly improved bioavailability and curative effect.
2020-09-02
02
2020-09
Approved "Sodium Phosphate Creatine for Injection"
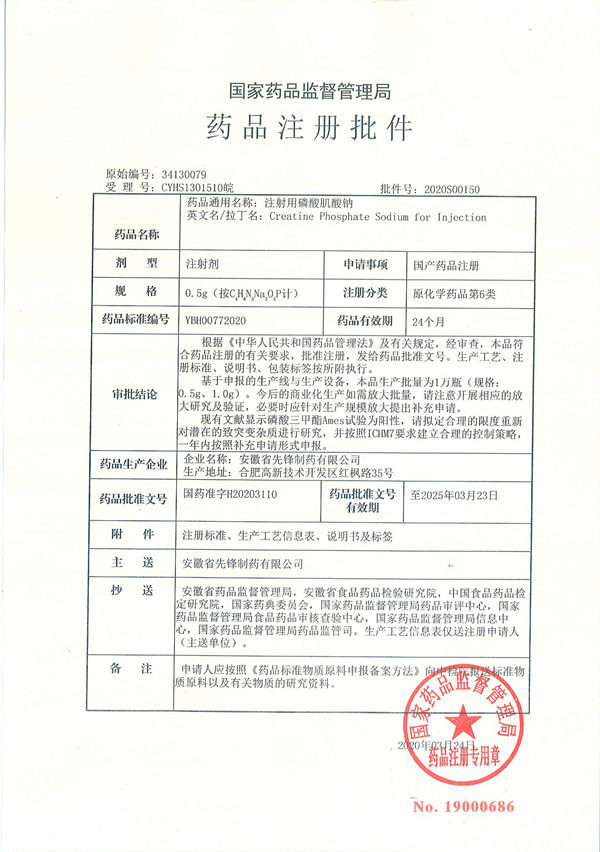
"Sodium creatine phosphate for injection" was approved" Creatine Phosphate Sodium for Injection is an injection declared by our company according to the 6 types of original chemical drugs. This product was established in 2010, and supplementary research was carried out in 2017. In 2019, we experienced on-site inspection. Under the leadership of Chairman Chen Zhenghao, The two major R&D centers of the company, Hefei and Shanghai, cooperated sincerely, and all links of R&D, production, quality, and procurement were united and united, and finally approved on March 24, 2020. This is another new milestone in the company's development history!
2020-09-02
02
2020-09
Anhui University of Traditional Chinese Medicine Anhui Pioneer Pharmaceutical Co., Ltd. Industry-university-research cooperation symposium On the afternoon of August 26, 2020, at the invitation of the leaders of the School of Pharmacy, Anhui University of Traditional Chinese Medicine, Chairman Chen Zhenghao of Anhui Pioneer Pharmaceutical Co., Ltd. and a group of people visited the company's Friendship College - School of Pharmacy, Anhui University of Traditional Chinese Medicine. At Anhui University of Traditional Chinese Medicine, Chairman Chen Zhenghao and his team were warmly received by the Secretary of the School of Pharmacy Wu Dawu, Dean Gui Shuangying and other leaders, and conducted in-depth exchanges on cooperation in various aspects such as industry-university-research, co-construction of laboratories and practice bases.
2020-09-02
01
2020-09
The 2020 (37th) National Pharmaceutical Industry Information Annual Conference will be held in Zhuhai, China on August 29, 2020
2020-09-01
26
2020-08
The company has reached strategic cooperation with Duzheng Biotechnology
On August 24, Anhui Pioneer Pharmaceutical Co., Ltd. and Changsha Duzheng Biotechnology Co., Ltd. (referred to as "Duzheng Bio") formally signed a strategic cooperation agreement. Chen Zhenghao, Chairman of Pioneer Pharmaceuticals, Ouyang Dongsheng, Chairman/President of Duzheng Bio, Li Xiaohui, Vice President and other leaders attended the signing ceremony.
2020-08-26
Contact us
Add:No.35 HongFeng Road, Hefei, Anhui, China.
Tel:86-551-65355028
Fax:86-551-65355022
Email:xfcn@xfcn.com

Follow us
Copyright © 2023 Anhui Pioneer Pharmaceutical Co., Ltd 皖ICP备2020016893号 Powered by:www.300.cn SEO










 86-551-65355028
86-551-65355028