关于先锋
Company Profile
Warmly congratulate Pioneer Pharmaceutical Clindamycin Phosphate Injection on obtaining the new approval document, new standard and new Ruijia for generic drug registration!
Release time:
2023-02-27
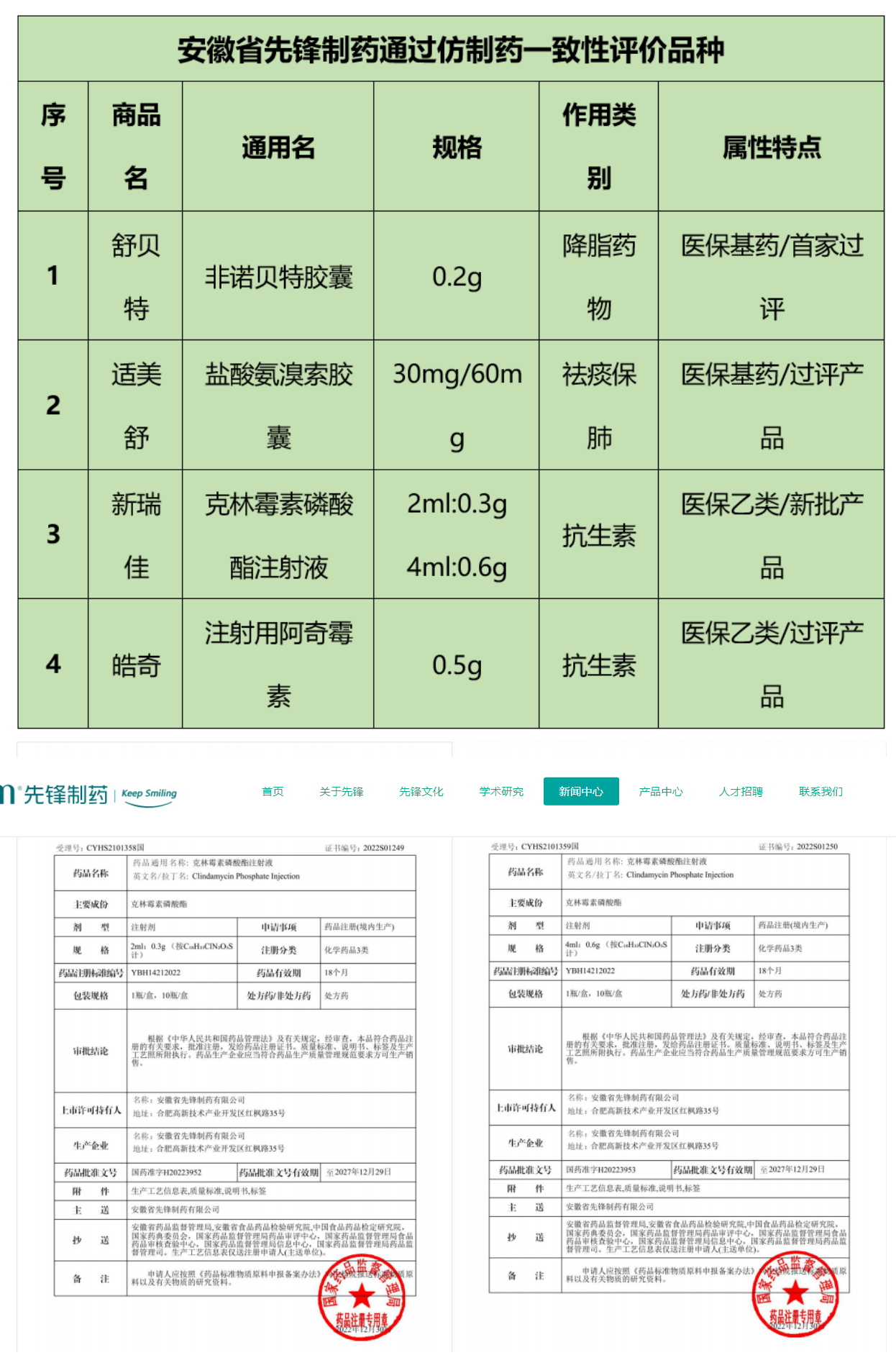
On the occasion of the New Year's Day in 2023 , Anhui Pioneer Pharmaceutical Co., Ltd. received two notices of approval for registration of generic drugs issued by the State Food and Drug Administration. The approved product is Clindamycin Phosphate Injection (Trademark: Xinruijia® ) with specifications of 2ml :0.3g and 4ml:0.6g ), and the approval numbers are Guoyao Zhunzi H20223952 and Guoyao Zhunzi H20223953 respectively . At the beginning of the new year, Pioneer Pharmaceuticals successfully obtained 2 R&D achievements, which will definitely have far-reaching significance for the long-term development of the company!
On the occasion of the New Year's Day in 2023 , Anhui Pioneer Pharmaceutical Co., Ltd. received two notices of approval for registration of generic drugs issued by the State Food and Drug Administration. The approved product is Clindamycin Phosphate Injection (Trademark: Xinruijia® ) with specifications of 2ml :0.3g and 4ml:0.6g ), and the approval numbers are Guoyao Zhunzi H20223952 and Guoyao Zhunzi H20223953 respectively . At the beginning of the new year, Pioneer Pharmaceuticals successfully obtained 2 R&D achievements, which will definitely have far-reaching significance for the long-term development of the company!
After the product was approved, the company started to implement the local industrialization of the product in time. From February 23rd to 28th , 2023 , the Provincial Food and Drug Administration has arranged a working group to enter Anhui Pioneer Pharmaceuticals to carry out new product production verification inspections ! New approvals, new standards, and Xinruijia will be on the market soon, so stay tuned!
Pioneer Pharmaceuticals in Anhui Province currently has 1 newly registered generic drug that is deemed to have been evaluated (clindamycin phosphate injection), and another 3 products that have passed the consistency evaluation of generic drug quality and efficacy (fenofibrate capsules, Ambroxol Hydrochloride Capsules, Azithromycin for Injection), sincerely invite new and old customers to discuss cooperation!
Related news
Contact us
Add:No.35 HongFeng Road, Hefei, Anhui, China.
Tel:86-551-65355028
Fax:86-551-65355022
Email:xfcn@xfcn.com

Follow us
Copyright © 2023 Anhui Pioneer Pharmaceutical Co., Ltd 皖ICP备2020016893号 Powered by:www.300.cn SEO




 86-551-65355028
86-551-65355028